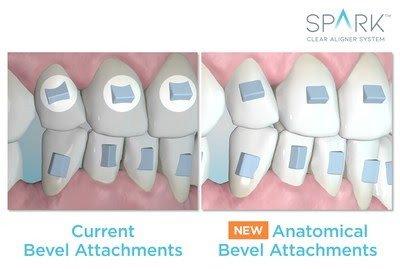
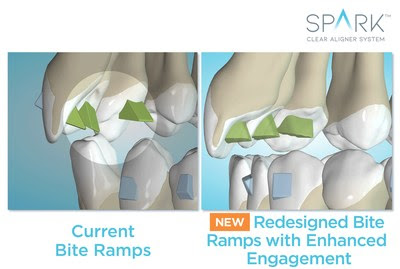
BREA, Calif., June 16, 2021 /PRNewswire/ — Ormco Corporation, a global leader of orthodontic solutions, today announced it has received U.S. Food and Drug Administration (FDA) clearance for the use of its Spark™ Clear Aligner System for orthodontic treatment in patients with both primary and secondary teeth (mixed dentition), providing the green light for orthodontists to treat their younger patients who can benefit from clear aligner therapy.
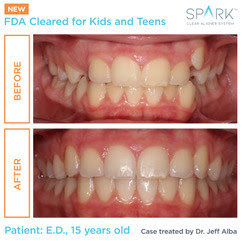
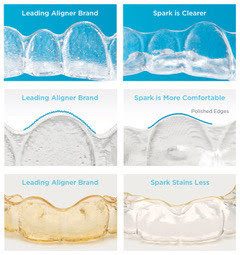
Designed to fit the lifestyle of today’s active kids and teens, Spark Clear Aligners are now one of the few brands approved by the FDA to safely and effectively treat younger patients with cases that range from simple to more complex. Spark Aligners are a doctor-directed treatment option that is clearer, more comfortable, and stains less than the leading aligner brand. In fact, 96% of patients prefer Spark Clear Aligners to the leading brand based on clarity and comfort.1 Spark Aligners have created thousands of healthy smiles worldwide since 2019.
 “My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!”
“My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!”
Spark Aligners are made with TruGENTM material, the latest innovation in proprietary branded aligner material. TruGEN resists stains better and is even clearer than the leading aligner material. The edges are polished and smoother to avoid the occasional discomfort that can result from other aligner brands. Unlike online or at-home aligner products that eliminate the important role of the orthodontic specialist, Spark Aligners involve an experienced doctor in every aspect of treatment.
 “My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.”
“My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.”
Doctors worldwide have successfully used Spark Aligners to treat a wide variety of patient orthodontic issues including open bite, deep bite, overbite, underbite, crossbite, crowding, spacing, semi-erupted teeth and extractions.
 “Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”
“Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”
Parents and other consumers can learn more about the Spark Clear Aligner System, view patient before & after images, and find a Spark Aligners doctor at sparkaligners.com
About the Spark™ Clear Aligner System
Spark™ Aligners are manufactured by Ormco™, a global leader in innovative orthodontics products, with 60 years of orthodontics expertise, R&D and high manufacturing standards. Ormco has helped doctors create more than 20 million smiles in more than 130 countries. Spark Aligners offer the latest advancements in clear aligners and a better patient experience. Spark Approver 3D application and advanced clear aligner technology with TruGEN™ material is designed to meet the needs of orthodontists, providing more efficient and effective tooth movement, compared to the leading aligner brand. Spark Aligners are designed to give orthodontists more control and flexibility so that they can more easily achieve healthy, confident smiles.
Trusted by orthodontists worldwide, Spark Aligners are clearer, more comfortable and stain less than the leading aligner brand. Spark Aligners are loved by patients: 100 percent of Spark patients surveyed would recommend Spark Clear Aligners to a friend.¹ For more information about Spark Aligners, visit www.sparkaligners.com.
*Dr. Bill Dischinger is a paid consultant of Ormco. The opinions expressed are those of Dr. Dischinger. Ormco is a medical device manufacturer and does not dispense medical advice. Clinicians should use their own professional judgment in treating their patients.
Basis for Release: Claims Matrix Form 045:
New Indications for Use: “The Spark ™ Clear Aligner System is indicated for the alignment of teeth during orthodontic treatment of malocclusion.” Formerly the indication was: “The Ormco Spark Clear Aligner System is indicated for the treatment of tooth malocclusion in patients with permanent dentition (i.e., all second molars). The Ormco Clear Aligner System positions teeth by way of continuous gentle force.
3. Spark™ Aligners are a nearly invisible, discrete aligner treatment system.
13. Spark Aligners are designed to provide a more comfortable and productive treatment.
17. Spark Clear Aligners are proven to have minimal aligner stains, which helps keep your smile shining during treatment.
20. TruGEN™ material is proven to provide advanced force retention compared the leading aligner material. Force retention has been found to result in more increased tooth movement. This may result in more efficient and effective tooth movement compared to the leading aligner material.
21. Spark Clear Aligners are designed to be clearer than the leading competitor.
32. “Doctors worldwide are using Spark Clear Aligners to treat a wide variety of patient malocclusions including open bite, deep bite, overbite, underbite, crossbite, crowding, spacing.”
33. 100% of patients surveyed would recommend Spark Aligners to a friend.
40. More clear. More comfortable.
41. Patients agree that Spark Aligners are more comfortable to wear.
MEDIA CONTACT:
Sharon Scott, [email protected]
310.594.6545
1 Data on file with Ormco™




 “My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!”
“My son and I have been so happy with his experience using Spark Aligners,” said Mrs. Strecker, a parent of a 9-year-old who needed treatment for spacing as well as AP correction. “Our orthodontist recommended Spark because he achieved outstanding results with other patients and told us the material used in Spark Aligners would be the clearest option while also being more comfortable than the leading competitive alternative. Because people could barely notice he was wearing aligners, my son felt confident during his entire treatment. We saw his smile change dramatically in about 3 months and treatment completion with a beautiful smile in 5 months. We are big Spark fans!” “My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.”
“My practice has been impressed with the results we are achieving for our patients with Spark Aligners,” said Dr. Bill Dischinger* of Dischinger Team Orthodontics. “The TruGEN material from which the aligners are made delivers better surface contact providing faster and more reliable tooth movements. With Spark Aligners, we’re seeing stronger and more predictable outcomes than we’ve achieved with other aligners, so the expanded indication of usage is great news for my younger patients who lead busy lifestyles filled with sports, music and other activities. My entire team and our patients are thrilled with the efficient, reliable treatment experience that Spark Aligners deliver.” “Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”
“Spark Aligners were made in collaboration with leading orthodontists. This along with TruGEN™, the most advanced material available, ensures a better treatment experience for patients,” said Sheila Tan, Vice President of Global Marketing & Clinical Education. “We are delighted with the FDA clearance to expand our treatment options to include kids and teens. Now Spark Aligners can be used to treat young children that do not have all of their permanent teeth.”